ISO 13485: 2016
The standard for Medical Devices
When you place medical devices on the European market, they must comply with European quality legislation and guidelines. Introducing a quality system according to EN ISO 13485 and ISO 14971 helps you with this. These standards, in which risk management plays an important role, have been specifically developed for medical device manufacturers. ISO 13485: 2016 is also in high demand from medical device manufacturers to companies that store medical devices, perform part of the production process or produce parts for a medical device. As an organization you are then a critical subcontractor of the legal manufacturer. ISO 13485 certification is not mandatory. However, the law requires the presence of a quality management system.
In addition to certification that this standard demonstrates that your processes are controlled and guaranteed, it is a very good basis for the Medical Device Regulation which will replace the Medical Device Directive in 2020.
The most important changes to the new ISO 13485: 2016 are;
• Definitions of, among others, the manufacturer, distributor and importer have been clarified so that it becomes clearer who is regarded by law as a legal manufacturer;
• Risk management and risk-based decision-making apply not only to design and development, but to all parts of the quality management system. The term risk has been added to the standard requirements in around 30 places;
• Additional requirements and clarity with regard to verification, validation and design activities; including the addition of design transfer as design activity (section 7.3.8) and the synergy with the FDA Device Master Record (DMR) indicated in section 4.2.3 .;
• Strengthening supplier control processes;
• Increased focus with regard to feedback mechanisms;
• More explicit requirements for software validation for different applications
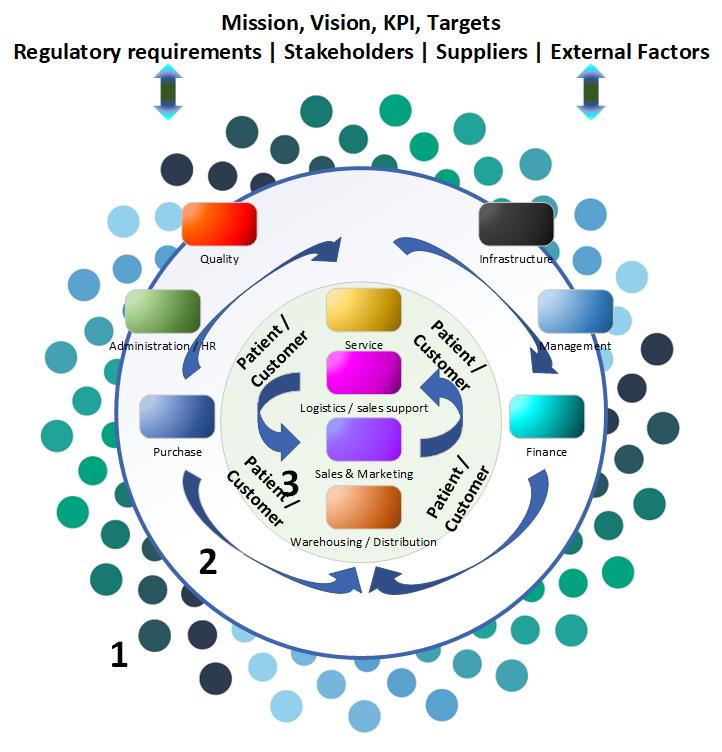
The transition period of ISO 13485: 2003 and ISO 13485: 2012 is until February 29, 2019. QMS Improvement can advise and guide you in setting up your QMS system in accordance with the EN ISO 13485: 2016 standard, whereby we use a modular application. Depending on your organization, clauses from the standard are applicable or may, if properly substantiated, be excluded. The image below shows an example of how the modular set-up works.
"The system is very skilfully designed, the modular application means that you as a growing organization can easily expand this system"Quote from Lead Auditor Bureau Veritas
Save time
Save time setting up your system by using our standard templates, risk analysis and corrective and preventive action templates.
Easy to edit
Thanks to our modular structure of your system, it remains easy to maintain, even as you grow
Friendly support
By staying in touch with each other, we know what is going on in your organization and we can continue to support you
Grow your business
See how your profit increases and your value increases as you boost the efficiency of your business. It's easier than you think.


